- How do I convert between moles and particles in a given chemical equation?
- What is the relationship between a mole and Avogadro's number when working with particles?
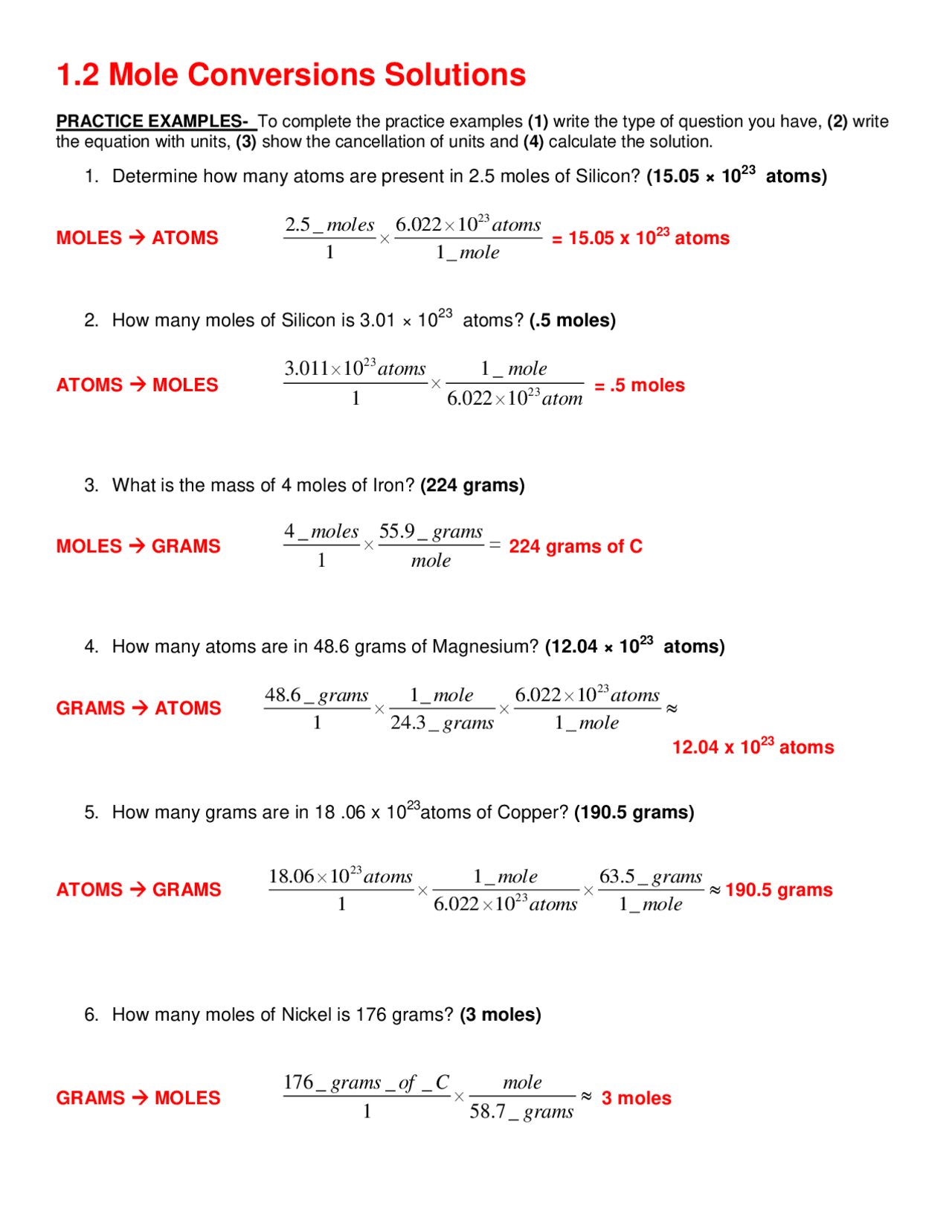
- Can you provide an example of a mole conversion worksheet for practicing mole-to-particle conversions?
- How can I use the concept of moles to determine the mass of a substance in a chemical reaction?
- Are there any specific strategies or tips for effectively working with moles and particles in mathematical calculations?
Understanding Moles and Particles: A Foundation for Mole Conversion
In this section, we will delve into the fundamental concepts of moles and particles, which form the basis for mole conversion calculations. By grasping the relationship between these two entities, students can confidently convert between different units and solve problems involving mole conversions.
The Mole Conversion Worksheet: Enhancing Problem-Solving Skills
The mole conversion worksheet serves as a valuable tool for students to practice applying their understanding of mole conversion concepts. Through a series of exercises and problems, students can enhance their problem-solving skills and develop proficiency in converting between moles and particles.
Step-by-Step Approach to Solving Mole Conversion Problems
This section outlines a step-by-step approach that students can follow to effectively solve mole conversion problems. By breaking down the problem-solving process into manageable steps, students can systematically convert between different units and ensure accurate results. Emphasize the importance of understanding the given information, setting up the conversion factors, and canceling units appropriately.
Common Pitfalls and Strategies for Success in Mole Conversion
This final section highlights common pitfalls that students may encounter when working with mole conversion problems and provides strategies for overcoming them. It emphasizes the significance of practicing regularly, identifying and rectifying mistakes, and seeking additional help or resources when needed. Highlight the importance of unit analysis and double-checking calculations to avoid errors.
frequently asked questions
How do I convert between moles and particles in a given chemical equation?
To convert between moles and particles in a chemical equation, use Avogadro's constant. One mole of any substance contains 6.022 x 10^23 particles. To convert from moles to particles, multiply the given amount by Avogadro's constant. To convert from particles to moles, divide the given amount by Avogadro's constant.
What is the relationship between a mole and Avogadro's number when working with particles?
A mole is a unit of measurement used in chemistry to represent the amount of a substance. Avogadro's number is a fundamental constant that represents the number of particles (atoms, molecules, or ions) in one mole of a substance. Therefore, there is a direct relationship between a mole and Avogadro's number when working with particles: 1 mole of any substance contains 6.022 x 10^23 particles.
Can you provide an example of a mole conversion worksheet for practicing mole-to-particle conversions?
Sure! Here is an example of a mole conversion worksheet for practicing mole-to-particle conversions:
1. Convert 3.5 moles of oxygen to the number of particles.
Answer: 2.105e24 particles
2. Convert 0.25 moles of sodium chloride to the number of formula units.
Answer: 1.5e23 formula units
3. Convert 2.8 moles of water to the number of molecules.
Answer: 1.6816e24 molecules
4. Convert 1.2 moles of carbon dioxide to the number of atoms.
Answer: 7.232e23 atoms
5. Convert 0.75 moles of ammonia to the number of molecules.
Answer: 4.505e23 molecules
Remember to use Avogadro's number (6.02214076 × 10^23) as a conversion factor when solving these problems.
How can I use the concept of moles to determine the mass of a substance in a chemical reaction?
The concept of moles can be used to determine the mass of a substance in a chemical reaction by using the molar mass. The molar mass is the mass of one mole of a substance, and it is expressed in grams per mole. By using the balanced chemical equation, which shows the ratio of moles between reactants and products, one can convert the given amount of a substance (in moles) to its mass (in grams) using the molar mass as a conversion factor.
Are there any specific strategies or tips for effectively working with moles and particles in mathematical calculations?
Yes, there are specific strategies and tips for effectively working with moles and particles in mathematical calculations. One important strategy is to understand the concept of a mole and its relationship to Avogadro's number. This allows you to convert between moles and particles. Additionally, it is crucial to use the molar mass of a substance to convert between grams and moles. It is also helpful to practice dimensional analysis, where conversion factors are used to cancel out units and ensure the correct unit is obtained in the final answer. Finally, it is essential to carefully read and interpret the given information in the problem and apply the appropriate mathematical formulas or equations.
In conclusion, the mole conversion worksheet provides a valuable tool for students to practice working with moles and particles in their mathematics education journey. By utilizing conversion factors and understanding the relationship between moles and particles, students can confidently convert between these units and solve complex problems. This worksheet serves as a hands-on exercise that promotes critical thinking, problem-solving skills, and strengthens their conceptual understanding of chemistry and mathematics. With continued practice and application of these concepts, students will be well-equipped to tackle more advanced topics in their studies.
If you want to know other articles similar to Moles & Particles: A Conversion Worksheet for Mole Calculations you can visit the category General Education.